Class 9 Science Is Matter Around Us Pure?
Introduction
In our everyday lives, we encounter various materials like water, air, food, and metals. But are these materials pure? When scientists talk about something being pure, they mean it is made of only one kind of particle. Pure substances have uniform composition and properties throughout. However, most of the matter around us is not pure; it is a mixture of different substances. For example, air is a mixture of gases like nitrogen, oxygen, and carbon dioxide. Understanding the difference between pure substances and mixtures helps us better understand the world around us.
Matter
Matter is anything that has mass and occupies space.
Matter can exist in three primary states.
- Solid: Has a definite shape and volume. The particles are closely packed together and vibrate in place.
- Liquid: Has a definite volume but takes the shape of its container. The particles are less tightly packed than in a solid and can move around each other.
- Gas: Has neither a definite shape nor a definite volume. The particles are far apart and move freely.
Pure Substance
A substance that is made up of only one kind of particle is called a pure substance.
Examples: Gold, Oxygen, Hydrogen, Water, Carbon dioxide etc…
Characteristics of Pure Substance
- A pure substance is homogenous.
- A pure substance has a definite set of properties.
- A pure substance can not be separated into another kind of matter by physical process.
There are two types of pure substances.
Elements
The substances that are made up of only one kind of particle and cannot be divided into simpler substances by chemical reactions are called elements.
Examples: Gold, Copper, Iron etc.
Compounds
The substances that are formed by the chemical combination of two or more elements in fixed ratios are called pure substances but can be broken down into simpler substances by chemical reactions.
Examples: Water, Carbon dioxide etc…
Properties of Compounds
- Elements in a compound combine together chemically in a fixed proportion.
- The compound can be separated into its constituent elements by chemical process.
- The properties of compounds are very different from their constituent elements.
Mixtures
Mixtures are impure substances that are formed by the physical combination of two or more elements or compounds in any ratio.
Example: Air, Water, Milk, Sand, Namkeen etc…
There are two main types of mixtures
Homogeneous Mixture
A mixture whose composition is uniform throughout is called a homogeneous mixture. The boundaries between its various components cannot be seen separately.
Example: Mixture of salt and water, mixtures of sugar and water
Heterogeneous Mixture
A mixture whose composition is not uniform and contains visibly distinct phases is called a heterogeneous mixture.
Example: Sand in water, polluted water.
Solution
A solution is a homogeneous mixture of two or more substances.
Example: Salt+Water, Aerated Drinks, Air, Tincher of Iodine etc…
It consists of two main components:
Solvent
The component present in larger quantity, which dissolves the other component(s), is called the solvent.
Solute
The component present in smaller quantity, which is dissolved in the solvent, is called the solute.
Colloidal Solution
A mixture in which particles of a substance are distributed evenly throughout the solution is called colloidal solution.
Particles of a colloid are larger than that of solution but smaller than those of suspension. Therefore a colloid appears to be homogenous but is actually heterogenous in nature.
Dispersed Phase: It is the solute-like component.
Dispersion Medium: It is the solvent-like component.
| Dispersed Phase | Dispersing Medium | Type | Example |
|---|---|---|---|
| Gas | Gas | Aerosol | Fog, clouds, mist |
| Liquid | Solid | Aerosol | Smoke, automobile exhaust |
| Liquid | Gas | Foam | Shaving cream |
| Liquid | Liquid | Emulsion | Milk, face cream |
| Solid | Liquid | Sol | Milk of magnesia, mud |
| Gas | Solid | Foam | Foam, rubber, sponge, pumice |
| Liquid | Liquid | Gel | Jelly, cheese, butter |
| Solid | Solid | Solid Sol | Coloured gemstone, milky glass |
Tyndall Effect
The scattering of a beam of light by a medium containing suspended particles is called the Tyndall effect after the name of the scientist who discovered this effect.
Tyndall effect can also be observed when a fine beam of light enters a room through a small hole. This happens due to the scattering of light by the particles of dust and smoke in the air.
Concentration of Solution
The concentration of a solution refers to the amount or mass of solute present in a given amount of solvent or solution at a certain temperature.
It can be calculated using the formula:
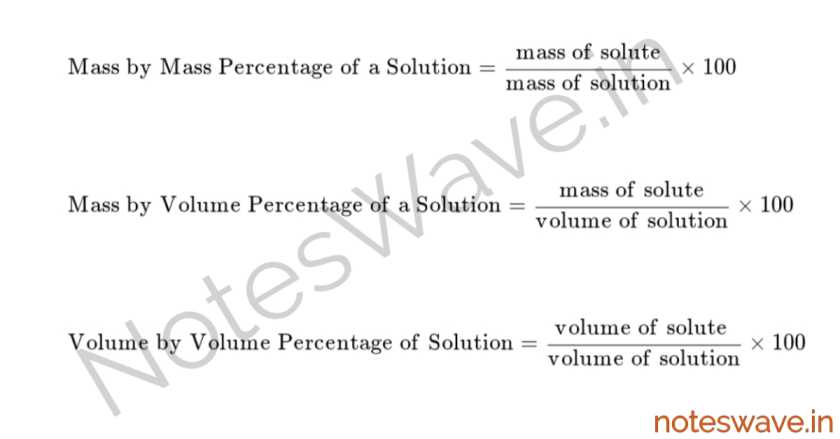
Saturated Solution
A solution in which no more solute can be dissolved at that particular temperature is called a saturated solution.
Unsaturated Solution
A solution in which more solute can be dissolved at that particular temperature,Example is called an unsaturated solution.
Solubility
The maximum amount of a solute which can be dissolved in 100 grams of a solvent at a specified temperature is known as the solubility of that solute in that solvent at that temperature.
Separation of Components from a Mixture
Various methods are used for separating components from a mixture:
- Vaporization: The process of transforming a substance into vapour at a temperature lower than its boiling point is called vaporization. This method is used to separate volatile components from non-volatile ones.
- Separation Funnel: Used for separating insoluble substances with different densities, such as oil and water.
- Sublimation: Used to separate sublimable substances from non-sublimable ones.
- Chromatography: Used to separate solutes dissolved in the same solvent.
- Distillation: Used to separate components with significantly different boiling points.
- Fractional Distillation: Specifically used for separating liquids with boiling points close to each other.
- Crystallization: Used to obtain pure solid crystals from a solution.
Physical Changes
Those changes in which no new substances are formed are called physical changes. In a physical change, the substances involved do not change their identity. They can be easily returned to their original form by some physical process. This means that physical changes can be generally reversed. The changes in physical state, size and shape of a substance are known as physical changes.
Example of Physical Change
- Melting of Ice: When ice melts, it changes from a solid to a liquid (water). The chemical composition (H₂O) remains the same.
- Boiling of Water: When water boils, it changes from a liquid to a gas (steam or water vapor). The chemical composition (H₂O) remains unchanged.
- Tearing Paper: When you tear a piece of paper, its size and shape change, but the paper itself remains paper.
- Dissolving Sugar in Water: When sugar dissolves in water, it disperses in the water, but its chemical composition (C₆H₁₂O₆) remains the same.
- Chopping Wood: When wood is chopped, it changes in size and shape, but the chemical composition of the wood remains the same.
Chemical Changes
Those changes in which new substances are formed are called chemical changes. In a chemical change, the substances involved change their identity. They get converted into entirely new substances. The new substances usually cannot be returned to their original form. This means that chemical changes are usually irreversible.
Example of Chemical Change
- Burning of Magnesium: When a magnesium wire is heated, it burns in air to form a white powder called magnesium oxide. This magnesium oxide is an entirely new substance. Thus, a new chemical substances is formed during the burning of a magnesium metal wire. So, the burning of a magnesium wire is a chemical change.
- Rusting of Iron: When iron reacts with oxygen in the presence of water or moisture, it forms iron oxide (rust), which is a new substance.
- e.g. 4Fe + 3O2 + 6H2O→4Fe(OH)3
- Vinegar and Baking Soda Reaction: When vinegar (acetic acid) reacts with baking soda (sodium bicarbonate), it produces carbon dioxide gas, water, and sodium acetate.
- e.g. CH3COOH + NaHCO3→CO2 + H2O + NaCH3COO
- Burning of Wood: When wood burns, it reacts with oxygen in the air to form carbon dioxide, water vapor, and ash.
- e.g. Wood + O2→CO2 + H2O + Ash
- Baking a Cake: When baking a cake, the ingredients (flour, sugar, eggs, etc.) undergo chemical reactions when heated, resulting in a new product with different properties.
| Check it 👇 |
|---|
| CBSE Class 9 Science Chapter 2 Is Matter Around Us Pure? Numericals ( Comming Soon ) |
| CBSE Class 9 Science Chapter 2 Is Matter Around Us Pure? MCQ’s (Comming Soon ) |
| CBSE Class 9 Science Chapter 2 Is Matter Around Us Pure? pdf link click here (coming soon) |
We hope that provided CBSE Class 9 Science Notes, “Is Matter Arround Us Pure?,” will be helpful to you. If you have any queries regarding the NCERT Class 9 Science Notes, please drop a comment below, and we will get back to you as soon as possible.
