Section A Biology ( 30 Marks)
| Q.N. | Section A | Marks |
|---|---|---|
| 1. | Select the group in which all organisms have the same mode of nutrition. A. Cuscuta, yeast, legumes, leeches and tapeworm B. Cactus, ticks, lice, leeches and cow C. Cuscuta, ticks, lice, leeches and tapeworm D. Cactus, grass, lice, lion and tapeworm | 1 |
| 2. | Which of the following options indicates the products formed after breakdown of the glucose in our muscle cells when there is lack of oxygen? A. Ethanol + carbon dioxide + Energy B. Lactic acid + Energy C. Lactic acid + carbon monoxide + Energy D. Carbon dioxide + Water + Energy | 1 |
| 3. | Which of the following is a correct combination of function and part of the brain? A. Posture and balance: Cerebrum B. Salivation: Medulla in midbrain C. Hunger: Pons in hindbrain D. Blood pressure: Medulla in hindbrain | 1 |
| 4. | The blood glucose level in a patient was very high. It may be due to inadequate secretion of: A. growth hormone from pituitary gland B. oestrogen from ovary C. insulin from pituitary gland D. insulin from pancreas | 1 |
| 5. | In a cross between black furred rabbit (B) and white furred rabbit (b), all offspring were found to have black fur. What can be inferred about the genetic makeup of the parent rabbits? A. BB X bb B. Bb X Bb C. Bb X bb D. bb X bb | 1 |
| 6. | Which are the correct statements related to ozone?(i) Ozone layer helps in increasing the UV radiations reaching earth.(ii) Ozone is a deadly poison.(iii) Ozone layer shields the earth from UV radiations.(iv) Ozone layer prevents UV rays which cause skin cancer.(v) Ozone is formed with the help of Chloroflurocarbons. A. (i), (ii), (iii) B. (ii), (iii), (iv) C. (iii), (iv), (v) D. (i), (iv), (v | 1 |
| 7. | Which of the following human activities has resulted in an increase of non-biodegradable substances? A. Organic farming B. Increase in tree plantation C. Use of plastic as packaging material D. Composting of kitchen waste | 1 |
| 8. | Assertion (A): Tallness of a pea plant is controlled by an enzyme. Reason (R): The gene for that enzyme makes proteins which help the plant to be tall. | 1 |
| 9. | Assertion (A): Vulture will always have the least amount of pesticides in a food chain. Reason (R): Vulture occupies the last trophic level and it gets only 10% of energy of the previous trophic level. | 1 |
| 10. | Unlike animals, plants do not have any excretory products as they do not eat food. Comment upon the statement with justification. | 2 |
| 11. | Students to attempt either option A or B. A. How many chambers are there in the heart of the following organisms? How is mixing of oxygenated and deoxygenated blood prevented in their body? (i) Fishes(ii) Humans OR B. Explain the mechanism by which the water is transported in plants? | 2 |
| 12. | About 100 acres of forest land was declared as Natural reserve park. The following organisms were predominant in the Natural reserve park:rabbit, frog, grass, fish, fox, water insects, zebra, peacock, snake, trees, bird, owl, insects, tiger, vulture, duck. Create a food web comprising two separate food chains with different producers by using the above data. | 2 |
| 13. | Draw and explain how the nerve cells help in transmission of impulses? | 3 |
| 14. | In a genetic experiment, plants with pure round green seeds (RRyy) were crossed with plants with wrinkled yellow seeds (rrYY). (i) Show the gametes formed when F1 was self-pollinated. (ii) A total of 144 seeds were produced which developed into saplings. Show the ratio in which these traits are independently inherited in these144 sapling. | 3 |
| 15. | Neha consumed boiled sweet potatoes and boiled eggs for breakfast. Help her to understand some steps in the process of digestion of the food taken by her by answering the questions given below. Attempt either subpart A or B. A. Which of these food items is rich in proteins? In which part of the alimentary canal is the digestion of this component initiated? Name the enzymes, conditions required and the glands associated with the digestion here. OR B. Which of these food items contains fats? How is it digested? C. Which of these food items is rich in starch? How is its digestion initiated? D. The figure given below represents parts of the human alimentary canal. Which of these parts will have the maximum amount of digested food as soon as the process of digestion is completed? For visually impaired students D. How will the digested food be taken up by the alimentary canal? | 4 |
| 16. | Attempt either option A or B. A. Puneet wanted to grow banana plants. (i) Based on your knowledge on plant reproduction should he opt for seeds or any alternate method of reproduction. Justify your answer. (ii) Offsprings of a banana plant usually show very little variation. What causes variation and are variations good or bad? Justify. OR B. Annie was conducting research on the number of fruits produced by watermelon under different conditions. She grew 25 watermelon plants each in both glass house A and B. She introduced pollinators in glass house A only. (i) What difference will she observe in the number of fruits produced in the two glass houses? Explain with reason. (ii) List 3 changes that will occur in a flower once it gets fertilized. | 5 |
Section B Chemistry ( 25 Marks)
| Q.N. | Section B | Marks |
|---|---|---|
| 17. | Which of the following equations represent redox reactions and what are the values for ‘p’ and ‘q’ in these equations? Equation 1: Fe2O3(s) + 2Al(s) Al2O3 (s) + p Fe(l) + heat Equation 2: 2C4H10(g) + 13O2(g) △ 8CO2(g)+ q H2O(g) A. Only equation 1 is a redox reaction, p =1 and q=3 B. Both equations 1 and 2 are redox reactions, p= 2 and q=4 C. Only equation 2 is a redox reaction, p= 2 and q= 10 D. Both equations 1 and 2 are redox reactions, p= 2 and q=10 | 1 |
| 18. | Four statements about the reactions of oxides with dilute hydrochloric acid and aqueous sodium hydroxide are listed. I. Aluminium oxide reacts with both dilute hydrochloric acid and aqueous sodium hydroxide. II. Calcium oxide reacts with dilute hydrochloric acid and aqueous sodium hydroxide. III. Zinc oxide reacts with both dilute hydrochloric acid and aqueous sodium hydroxide. IV. Sulphur dioxide does not react with either dilute hydrochloric acid or aqueous sodium hydroxide. Which statements are correct? A. I and II B. I and III C. II and IV D. III and IV | 1 |
| 19. | An iron nail is added to each of the two test tubes ‘P’ and ‘Q’ containing aqueous copper (II) sulphate, and aqueous silver nitrate respectively. Which of the following observation is correct? A. In test tube ‘P’ iron nail is coated with a blue coating and in test tube ‘Q’ there is no reaction. B. Iron nail is coated with a brown coating in test tube ‘P’ and silver coating in test tube ‘Q’. C. There is no reaction in either of the test tubes ‘P’ or ‘Q’. D. There is no reaction in test tube ‘P’ but a silver coating on iron nail is seen in test tube ‘Q’. | 1 |
| 20. | Methyl orange is added to dilute hydrochloric acid and to aqueous sodium hydroxide. What is the colour of the methyl orange in each solution? (Table) | 1 |
| 21. | Which of the following substances when dissolved in equal volume of water, will have the highest pH value?A. Sulphuric acidB. Acetic acidC. Magnesium hydroxideD. Sodium hydroxide | 1 |
| 22. | When excess of carbon dioxide is passed through lime water, the milkiness disappears because A. water soluble calcium carbonate converts to water soluble calcium bicarbonate. B. insoluble calcium carbonate converts to water soluble calcium bicarbonate. C. water soluble calcium carbonate converts to insoluble calcium bicarbonate. D. insoluble calcium carbonate converts to insoluble calcium bicarbonate. | 1 |
| 23. | In the reaction of aqueous solution of barium chloride with aqueous solution of sodium sulphate, the aqueous solution formed will be: A. BaCl2 B. BaSO4 C. Na2SO4 D. NaCl | 1 |
| 24. | Assertion (A): C4H8, C4H6 and C4H10 are members of the same homologous series Reason (R): C4H8, C4H6, C3H4, C3H6, C2H4, C2H2 are unsaturated hydrocarbons. | 1 |
| 25. | The following activity is set-up in the science lab by the teacher.He clamped an aluminium wire on a stand and fixed a pin to the free end of the wire using wax. Then he heated the wire with a burner from the end where the wire is clamped. Students observed the pin fall off. (Image) A. If the teacher replaces aluminium wire by silver wire, will the students’ observation change? Justify your answer. B. Will the aluminium wire melt? Give reason for your answer. | 2 |
| 26. | Attempt either option A or B. A. An element ‘X’ is stored in kerosene, and cannot be extracted from its ore using a reducing agent. ‘X’ forms an ionic compound on reaction with chlorine. (i) Can we store ‘X’ in water? Give reason to support your answer. (ii) Identify element ‘X’. Name the process used and write the equation for extraction of ‘X’ from its ore. OR B. The domes of many building in Europe are made of copper. These domes now appear greenish in colour. (i) Why do the domes appear greenish though copper is orange-red in colour? (ii) In your opinion, should the copper domes be replaced by iron domes to overcome the problem of change of colour of copper domes? (iii) Domes used to be made from thin sheets of metals. Why did the ancient architects use copper to make domes | 3 |
| 27. | Amrita electrolysed distilled water using the set-up shown in figure 1. She was expecting two gases to be evolved at the anode and cathode respectively Figure 1 Suddenly, she realised that the bulb in the circuit did not glow when she used distilled water (figure 2) Figure 2 After this realization, she added a substance to the distilled water for electrolysis to take place. Answer the following questions based on the information given above: A. Which gas was she expecting to be formed at the anode and which one at the cathode respectively? B. Why did the bulb not glow when Amrita passed electricity through distilled water? C. Which substance was added by Amrita to distilled water to get the expected result? For visually impaired students Identify the type of reaction: A. ZnO + C Zn + CO B. ZnCO3 heat ZnO + CO2 C. 2Mg + O2 2 MgO + heat | 3 |
| 28. | Sara took 2 mL of dilute NaOH solution in a test tube and added two drops of phenolphthalein solution to it. The solution turned pink in colour. She added dilute H2SO4 to the above solution drop by drop until the solution in the test tube became colourless. 40 drops of dilute H2SO4 were used for the change in colour from pink to colourless. When Sara added a drop of NaOH to the solution, the colour changed to back to pink again. Sara now tried the activity with different volumes of NaOH and recorded her observation in the table given below: (Table) Answer the following questions based on the above information: A. If Sara used concentrated H2SO4 in place of dilute H2SO4, how many drops will be required for the change in colour to be observed? (a) 40 (b) < 40 (c) >40 Justify your answer. B. Sara measured 20 drops of dil. H2SO4 and found its volume to be 1 mL. If Sara observed a change in colour of NaoH solution by using 3 mL of H2SO4, how many mL of NaOH did she add to the test tube initially? OR Sara takes 10 drops of dilute H2SO4 in the test tube and adds two drops of phenolphthalein solution to it. Then she adds NaOH dropwise. Sara observes a change in colour after adding 20 drops of NaOH. What change in colour would she observe and why? C. Write a balanced chemical equation for the reaction taking place in the above experiment. Which of the following is true and why? The reaction is a (a) neutralisation and double displacement reaction (b) neutralisation and precipitation reaction (c) precipitation and double displacement reaction (d) neutralisation, double displacement as well as precipitation reaction. | 4 |
| 29. | Attempt either option A or B. A. A hydrocarbon with the formula CxHy undergoes complete combustion as shown in the following equation: 2CxHy + 9O2 → 6CO2 + 6H2O. (a) What are the values of ‘x’ and ‘y’? (b) Give the chemical (IUPAC) name of the hydrocarbon. (c) Draw its electron dot structure. (d) Name the alcohol which on heating with conc. H2SO4 will produce the above hydrocarbon CxHy. (e) Write a balanced chemical equation for the reaction of CxHy with hydrogen gas in presence of Nickel. OR B. The electronic structures of atoms P and Q are shown below (Figure) Based on the information given above, answer the following questions: (a) If P and Q combine to form a compound, what type of bond is formed between them? (b) Give the chemical formula of the compound formed. (c) The compound so formed is dissolved in water. Is the resultant solution acidic or basic in nature? Justify your answer. (d) Write the chemical equation for the reaction between ‘Q’ and ethanol. (e) What will be the formula of the compound formed when ‘P’ undergoes bonding with carbon? For visually impaired students A. A hydrocarbon with the formula CxHy undergoes complete combustion as shown in the following equation: 2CxHy + 9O2 → 6CO2 + 6H2O. (a) What are the values of ‘x’ and ‘y’?(b) Give the chemical (IUPAC) name of the hydrocarbon.(c) Is CxHy a saturated or an unsaturated hydrocarbon?(d) Name the alcohol which on heating with conc. H2SO4 will produce the above hydrocarbon CxHy.(e) Write a balanced chemical equation for the reaction of CxHy with hydrogen gas in presence of Nickel. OR B. Oxygen can combine with both metals and non-metals. It combines with Calcium to form CaO and with carbon to form CO2. (a) What type of bond is formed between carbon and oxygen? (b) Identify the type of bond formed between Calcium and oxygen. (c) Which of the above compounds will be a good conductor of electricity in molten state and why? (d) Comment on the physical state (solid, liquid or gas) of CaO and CO2. (e) What is the valency of carbon in CO2? | 5 |
Section C Biology ( 25 Marks)
| Q.N. | Section C | Marks. |
|---|---|---|
| 30. | Arnav was making notes and he wrote down the following statements from his understanding of reflection from curved surfaces. I. Concave mirrors can produce both real and virtual images depending on the position of the object. II. Convex mirrors always produce real, inverted images regardless of the object’s position. III. In both concave and convex mirrors, the image location can be determined using the mirror formula 1/f=1/v+1/u where f is the focal length, v is the image distance, and u is the object distance.Choose from the following the correct option that lists the correct statements about reflection from curved surfaces. A. I and II B. I, II and III C. II and III D. I and III | 1 |
| 31. | Choose the correct option from the below which explains the reason for us to perceive the day sky as blue. A. As sunlight passes through the atmosphere, shorter wavelengths, such as blue are scattered more than other colors. B. The sky appears blue because all colors are scattered equally, but blue light is stronger and more visible to the human eye. C. The blue color of the sky is due to longer wavelengths like red and orange scattering more than shorter wavelengths, making blue stand out more. D. The atmosphere contains blue-colored particles that give the sky its blue appearance. | 1 |
| 32. | Assertion (A): A point object is placed at a distance of 26 cm from a convex mirror of focal length 26 cm. The image will not form at infinity.Reason (R): For above given system the equation 1/f=1/v+1/u gives v =∞ | 1 |
| 33. | (Image) The above image shows the formation of an image with an optical instrument. A. Identify the optical instrument (shown schematically as a rectangle) in the image. B. What type of image is formed in this case? C. Based on the measurements given in the image, calculate the focal length of the instrument. …………………….. For visually impaired students A. Under what conditions can a convex lens form a virtual image? B. Why does a piece of paper catch fire if we allow sunlight to pass through a convex lens onto the paper? | 2 |
| 34. | Attempt either option A or B. (Figure) Find out the following in the electric circuit given in the figure- (i) Effective resistance of two 8 ohm resistors in the combination. (ii) Current flowing through the 4-ohm resistor OR Study the circuit and find out- (i) Current in 12 ohm resistor (ii) Difference in the readings of ammeter A1 and A2 if any …………………………. For visually impaired students A. You are given four resistors each having resistance of R ohm. Find the maximum and minimum resistance that can be made with these four resistors. OR B. A copper wire has a length L=2 m, a cross-sectional area A=0.5 mm2, and resistivity ρ=1.7×10−8 Ω-m. Calculate the resistance of another wire made of the same material whose length is twice the length of the wire but has the same cross-sectional area. | 2 |
| 35. | 3 | |
| 36. | A student needs to make a 0.12 Ω resistor. She has some copper wire of 0.80 mm diameter. Resistivity of copper is 1.8 × 10–8 Ωm (i) Determine the cross-sectional area of the wire. (ii) Calculate the length of wire required for the 0.12 Ω resistor. | 3 |
| 37. | 3 | |
| 38. | 4 | |
| 39. | 5 |
Section A Biology
1. Select the group in which all organisms have the same mode of nutrition.
A. Cuscuta, yeast, legumes, leeches and tapeworm
B. Cactus, ticks, lice, leeches and cow
C. Cuscuta, ticks, lice, leeches and tapeworm
D. Cactus, grass, lice, lion and tapeworm
C. Cuscuta, ticks, lice, leeches and tapeworm; as all of these are parasites.
2. Which of the following options indicates the products formed after breakdown of the glucose in our muscle cells when there is lack of oxygen?
A. Ethanol + carbon dioxide + Energy
B. Lactic acid + Energy
C. Lactic acid + carbon monoxide + Energy
D. Carbon dioxide + Water + Energy
B. Lactic acid + Energy
3. Which of the following is a correct combination of function and part of the brain?
A. Posture and balance: Cerebrum
B. Salivation: Medulla in midbrain
C. Hunger: Pons in hindbrain
D. Blood pressure: Medulla in hindbrain
D. Blood pressure: Medulla in hindbrain
4. The blood glucose level in a patient was very high. It may be due to inadequate secretion of:
A. growth hormone from pituitary gland
B. oestrogen from ovary
C. insulin from pituitary gland
D. insulin from pancreas
D. insulin from pancreas
5. In a cross between black furred rabbit (B) and white furred rabbit (b), all offspring were found to have black fur. What can be inferred about the genetic makeup of the parent rabbits?
A. BB X bb
B. Bb X Bb
C. Bb X bb
D. bb X bb
A. BB x bb
6. Which are the correct statements related to ozone?
(i) Ozone layer helps in increasing the UV radiations reaching earth.
(ii) Ozone is a deadly poison.
(iii) Ozone layer shields the earth from UV radiations.
(iv) Ozone layer prevents UV rays which cause skin cancer.
(v) Ozone is formed with the help of Chloroflurocarbons.
A. (i), (ii), (iii)
B. (ii), (iii), (iv)
C. (iii), (iv), (v)
D. (i), (iv), (v)
B. (ii), (iii), (iv)
7. Which of the following human activities has resulted in an increase of non-biodegradable substances?
A. Organic farming
B. Increase in tree plantation
C. Use of plastic as packaging material
D. Composting of kitchen waste
C. Use of plastic as packaging material.
8. Assertion (A): Tallness of a pea plant is controlled by an enzyme.
Reason (R): The gene for that enzyme makes proteins which help the plant to be tall.
A. Both A and R are true, and R is the correct explanation of A.
9. Assertion (A): Vulture will always have the least amount of pesticides in a food chain.
Reason (R): Vulture occupies the last trophic level and it gets only 10% of energy of the previous trophic level.
D. A is false but R is true
10. Unlike animals, plants do not have any excretory products as they do not eat food. Comment upon the statement with justification.
It is completely wrong to say that plants do not produce any excretory products. However, plants use completely different strategies for excretion than those of the animals.They get rid of these wastes in different manner (any two):
i. Oxygen, a photosynthetic waste, is removed through stomata.
ii. Excess water is removed by transpiration through stomata.
iii. Other metabolic wastes are either stored in dead cells, resins and gums or are removed through falling of old leaves.
iv. Many waste products are stored in cellular vacuoles
11. Students to attempt either option A or B.
A. How many chambers are there in the heart of the following organisms? How is mixing of oxygenated and deoxygenated blood prevented in their body?
(i) Fishes
(ii) Humans
OR
B. Explain the mechanism by which the water is transported in plants?
A.
(i) There are two chambers in the heart of fish. The blood is pumped to
the gills, is oxygenated there and passes directly to the rest of the
body.
(ii) There are four chambers in the heart of a human being. Separation
of the right side and the left side of the heart by septum prevents
mixing of oxygenated and de-oxygenated bloods
OR
B. Xylem moves water and minerals obtained from the soil through roots to all other parts of the plant in a unidirectional manner// Transpiration takes place from leaf which causes a transpirational pull in the tracheids and vessels of xylem facilitating upward movement of water// roots actively uptake ions from the soil, leading to difference in concentration gradient, thereby water moves into the roots to eliminate this difference/ creating a steady movement of water into root xylem.
12. About 100 acres of forest land was declared as Natural reserve park. The following organisms were predominant in the Natural reserve park: rabbit, frog, grass, fish, fox, water insects, zebra, peacock, snake, trees, bird, owl, insects, tiger, vulture, duck. Create a food web comprising two separate food chains with different producers by using the above data.
Tree food chain- tree, zebra, tiger /Any other food chain Grassland food chain- grass, zebra, tiger / Any other food chain Food web- Join the two food chains at a common point (zebra)
13. Draw and explain how the nerve cells help in transmission of impulses?
- All information from our environment is detected by the specialised tips of some nerve cells. The information acquired at the end of the dendritic tip of a nerve cell (Fig. a), sets off a chemical reaction that creates an electrical impulse.
- This impulse travels from the dendrite to the cell body, and then along the axon to its end.At the end of the axon, the electrical impulse sets off the release of some chemicals.
- These chemicals cross the gap, or synapse, and start a similar electrical impulse in a dendrite of the next neuron. This is how nervous impulses travel in the body. (Fig b).
14. In a genetic experiment, plants with pure round green seeds (RRyy) were crossed with plants with wrinkled yellow seeds (rrYY).
(i) Show the gametes formed when F1 was self-pollinated.
(ii) A total of 144 seeds were produced which developed into saplings. Show the ratio in which these traits are independently inherited in these144 sapling.
A. RY, Ry, rY, ry
B. The traits which are independently inherited are as follows
Tall round: 81
Tall wrinkled: 27
Short round: 27
Short wrinkled: 9
(Ratio :- 9 : 3 : 3 : 1)
15. Neha consumed boiled sweet potatoes and boiled eggs for breakfast. Help her to understand some steps in the process of digestion of the food taken by her by answering the questions given below.
Attempt either subpart A or B.
A. Which of these food items is rich in proteins? In which part of the alimentary canal is the digestion of this component initiated? Name the enzymes, conditions required and the glands associated with the digestion here.
OR
B. Which of these food items contains fats? How is it digested?
C. Which of these food items is rich in starch? How is its digestion initiated?
D. The figure given below represents parts of the human alimentary canal. Which of these parts will have the maximum amount of digested food as soon as the process of digestion is completed?
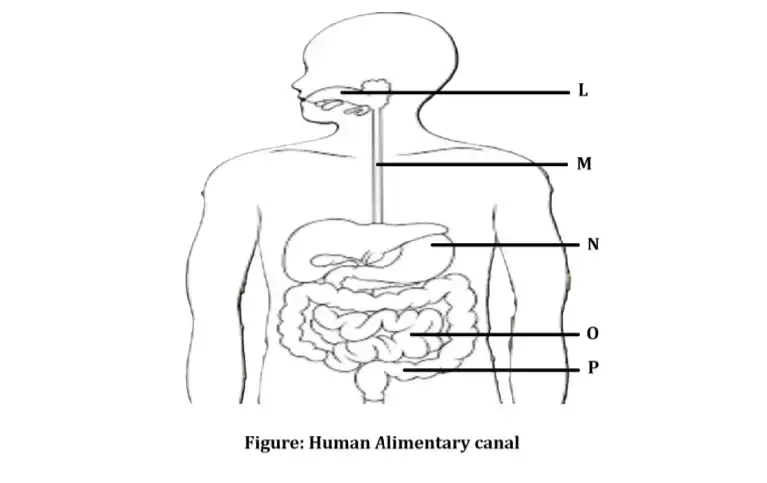
For visually impaired students
D. How will the digested food be taken up by the alimentary canal?
Students to attempt either subpart A or B.
A. Eggs are rich in proteins. The digestion of proteins is initiated in the stomach. Gastric glands present in the wall of the stomach release hydrochloric acid, a protein digesting enzyme called pepsin and mucus. The hydrochloric acid creates an acidic medium which facilitates the action of enzyme pepsin.
OR
B. Eggs contain fats. Bile juice from the liver breaks down large fat globules into smaller ones for increasing the efficiency of the enzymes and making the medium alkaline. Emulsified fats are digested by lipase secreted by pancreas.
C. Sweet potatoes are rich in starch. The saliva secreted by salivary glands present in buccal cavity contain an enzyme called salivary amylase that breaks down starch which is a complex molecule to give sugar.
D. Small Intestine will have a maximum amount of digested food as the process of digestion is completed in the small intestine.
For Visually impaired students
D. The digested food is taken up by the inner lining of the intestine with the help of finger-like projections or villi which increase the surface area for the absorption.
16. Attempt either option A or B.
A. Puneet wanted to grow banana plants.
(i) Based on your knowledge on plant reproduction should he opt for seeds or any alternate method of reproduction. Justify your answer.
(ii) Offsprings of a banana plant usually show very little variation. What causes variation and are variations good or bad? Justify.
OR
B. Annie was conducting research on the number of fruits produced by watermelon under different conditions. She grew 25 watermelon plants each in both glass house A and B. She introduced pollinators in glass house A only.
(i) What difference will she observe in the number of fruits produced in the two glass houses? Explain with reason.
(ii) List 3 changes that will occur in a flower once it gets fertilized.
Student to attempt either option A or B.
(i) Puneet should not choose seeds as banana plants have lost the capacity to produce seeds. He should go for vegetative propagation of banana (by stem cutting).
(ii) Errors and variations in DNA copying cause variation. Variation is good as it can help a population tide over unfavourable conditions by survival of some variants. It is bad as parents’ desirable characters are lost/ sometimes variants are not able to survive in the new conditions/ the variant is not able to use the cellular apparatus efficiently.
OR
(i) Watermelon has unisexual flowers, the male and female flowers are separate. The presence of pollinators will facilitate cross pollination between the flowers increasing the chance of fertilization and number of fruits being produced. Without pollinators the probability of pollen falling on stigma reduces in a unisexual flower, especially if they are far apart thus the number of fruits produced will be less.
(ii) The three changes observed are:
● Ovule develops a tough coat and becomes seed.
● Ovary grows and ripens to form fruit.
● Petals, sepals, stamen, style and stigma may shrivel and fall off
Section B Chemistry
17. Which of the following equations represent redox reactions and what are the values for ‘p’ and ‘q’ in these equations?
Equation 1: Fe2O3(s) + 2Al(s) —–>Al2O3 (s) + p Fe(l) + heat
Equation 2: 2C4H10(g) + 13O2(g) –△–> 8CO2(g)+ q H2O(g)
A. Only equation 1 is a redox reaction, p =1 and q=3
B. Both equations 1 and 2 are redox reactions, p= 2 and q=4
C. Only equation 2 is a redox reaction, p= 2 and q= 10
D. Both equations 1 and 2 are redox reactions, p= 2 and q=10
D. Both equations 1 and 2 are redox reactions, p= 2 and q=10
18. Four statements about the reactions of oxides with dilute hydrochloric acid and aqueous sodium hydroxide are listed.
I. Aluminium oxide reacts with both dilute hydrochloric acid and aqueous sodium hydroxide.
II. Calcium oxide reacts with dilute hydrochloric acid and aqueous sodium hydroxide.
III. Zinc oxide reacts with both dilute hydrochloric acid and aqueous sodium hydroxide.
IV. Sulphur dioxide does not react with either dilute hydrochloric acid or aqueous sodium hydroxide. Which statements are correct?
A. I and II
B. I and III
C. II and IV
D. III and IV
B. (I) and (III)
19. An iron nail is added to each of the two test tubes ‘P’ and ‘Q’ containing aqueous copper (II) sulphate, and aqueous silver nitrate respectively. Which of the following observation is correct?
A. In test tube ‘P’ iron nail is coated with a blue coating and in test tube ‘Q’ there is no reaction.
B. Iron nail is coated with a brown coating in test tube ‘P’ and silver coating in test tube ‘Q’.
C. There is no reaction in either of the test tubes ‘P’ or ‘Q’.
D. There is no reaction in test tube ‘P’ but a silver coating on iron nail is seen in test tube ‘Q’.
B. Iron nail is coated with a brown coating in test tube ‘P’ and silver coating
in test tube ‘Q’.
20. Methyl orange is added to dilute hydrochloric acid and to aqueous sodium hydroxide. What is the colour of the methyl orange in each solution?
| Sample | color in dilute hydrochloric acid | colour in aqueous sodium hydroxide |
| A | Orange | Red |
| B | Red | Yellow |
| C | Red | Orange |
| D | Yellow | Red |
| B. | Red | Yellow |
21. When excess of carbon dioxide is passed through lime water, the milkiness disappears because
A. water soluble calcium carbonate converts to water soluble calcium bicarbonate.
B. insoluble calcium carbonate converts to water soluble calcium bicarbonate.
C. water soluble calcium carbonate converts to insoluble calcium bicarbonate.
D. insoluble calcium carbonate converts to insoluble calcium bicarbonate.
D. Sodium hydroxide
22. When excess of carbon dioxide is passed through lime water, the milkiness disappears because
A. water soluble calcium carbonate converts to water soluble calcium bicarbonate.
B. insoluble calcium carbonate converts to water soluble calcium bicarbonate.
C. water soluble calcium carbonate converts to insoluble calcium bicarbonate.
D. insoluble calcium carbonate converts to insoluble calcium bicarbonate.
B. insoluble calcium carbonate converts to water soluble calcium
bicarbonate.
23. In the reaction of aqueous solution of barium chloride with aqueous solution of sodium sulphate, the aqueous solution formed will be:
A. BaCl2
B. BaSO4
C. Na2SO4
D. NaCl
D. NaCl
The following question consists of two statements – Assertion (A) and Reason (R). Answer these questions by selecting the appropriate option given below:
A. Both A and R are true, and R is the correct explanation of A.
B. Both A and R are true, and R is not the correct explanation of A.
C. A is true but R is false.
D. A is false but R is true
24. Assertion (A): C4H8, C4H6 and C4H10 are members of the same homologous series
Reason (R): C4H8, C4H6, C3H4, C3H6, C2H4, C2H2 are unsaturated hydrocarbons.
D. A is false but R is true
25. The following activity is set-up in the science lab by the teacher.He clamped an aluminium wire on a stand and fixed a pin to the free end of the wire using wax. Then he heated the wire with a burner from the end where the wire is clamped. Students observed the pin fall off.
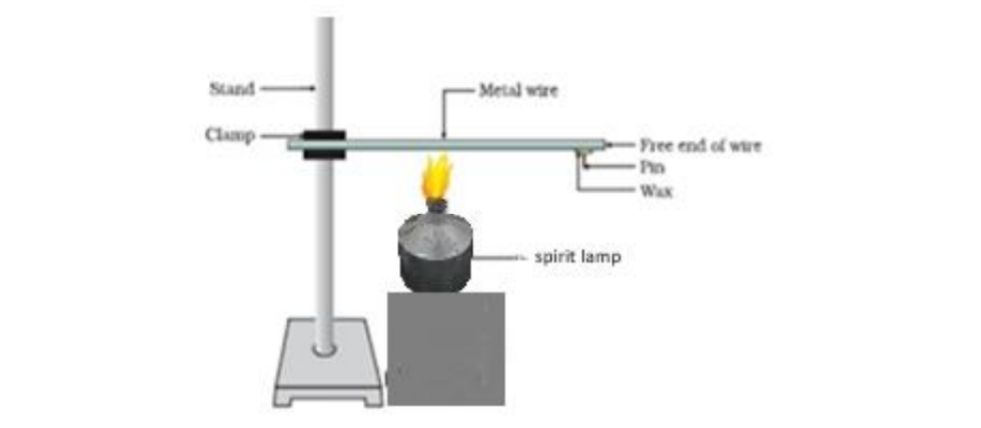
A. If the teacher replaces aluminium wire by silver wire, will the students’ observation change?
Justify your answer.
B. Will the aluminium wire melt? Give reason for your answer.
A. The pin will drop but will take less time to drop because silver is a better conductor of heat than aluminium.
B. No, aluminium wire will not melt because metals have high melting points.
26. Attempt either option A or B. A. An element ‘X’ is stored in kerosene, and cannot be extracted from its ore using a reducing agent. ‘X’ forms an ionic compound on reaction with chlorine.
(i) Can we store ‘X’ in water? Give reason to support your answer.
(ii) Identify element ‘X’. Name the process used and write the equation for extraction of ‘X’ from its ore.
OR
B. The domes of many building in Europe are made of copper. These domes now appear greenish in colour.
(i) Why do the domes appear greenish though copper is orange-red in colour?
(ii) In your opinion, should the copper domes be replaced by iron domes to overcome the problem of change of colour of copper domes?
(iii) Domes used to be made from thin sheets of metals. Why did the ancient architects use copper to make domes
Attempt either option A or B.
A.
(i) No, ‘X’ is highly reactive and will catch fire.
(ii) Sodium.
It is extracted from molten sodium chloride by electrolytic reduction
Cathode: Na+ + e– —> Na
Anode: 2Cl– —> Cl2 + 2e–
(Potassium is also a correct option)
OR
B.
(i) Copper gets oxidised/corroded to basic copper carbonate which is greenish in colour.
(ii) No, iron will rust and the reddish layer of rust will come off exposing iron to air, the dome will not be stable. Copper on the other hand on corrosion forms a protective layer which does not allow further corrosion.
(iii) Copper is a highly malleable metal, its thin sheets can be used to give different shapes of roofs, like the shape of a dome.
27. Amrita electrolysed distilled water using the set-up shown in figure 1. She was expecting two gases to be evolved at the anode and cathode respectively
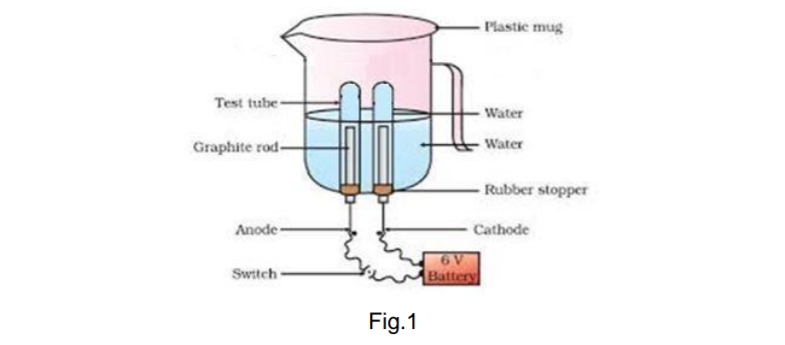
Suddenly, she realised that the bulb in the circuit did not glow when she used distilled water (figure 2)
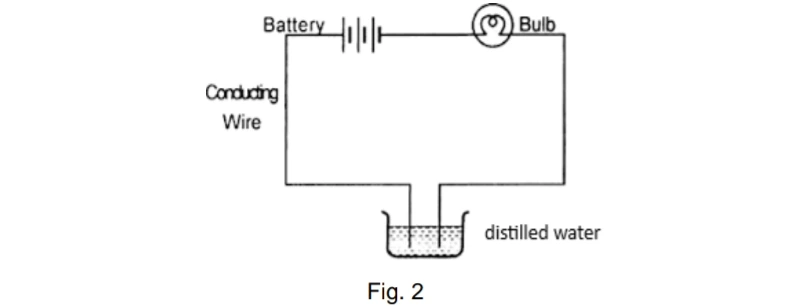
After this realization, she added a substance to the distilled water for electrolysis to take place. Answer the following questions based on the information given above:
A. Which gas was she expecting to be formed at the anode and which one at the cathode respectively?
B. Why did the bulb not glow when Amrita passed electricity through distilled water?
C. Which substance was added by Amrita to distilled water to get the expected result?
For visually impaired students
Identify the type of reaction:
A. ZnO + C —->Zn + CO
B. ZnCO3 —heat—> ZnO + CO2
C. 2Mg + O2 —-> 2 MgO + heat
A. She was expecting Oxygen gas to be formed at the anode and hydrogen at the cathode.
B. Distilled water is a poor conductor of electricity.
C. Adding few drops of H2SO4 or some NaCl (or any other strong electrolyte).
For visually impaired students
A. Redox reaction
B. Decomposition reaction and endothermic reaction
C. Combination reaction and exothermic reaction
28. Sara took 2 mL of dilute NaOH solution in a test tube and added two drops of phenolphthalein solution to it. The solution turned pink in colour. She added dilute H2SO4 to the above solution drop by drop until the solution in the test tube became colourless. 40 drops of dilute H2SO4 were used for the change in colour from pink to colourless. When Sara added a drop of NaOH to the solution, the colour changed to back to pink again. Sara now tried the activity with different volumes of NaOH and recorded her observation in the table given below:
| S. No. | Volume of dil. NaOH taken (mL) | Drops of dil. H2SO4 used |
| 1 | 2 | 20 |
| 2 | 3 | 30 |
| 3 | 4 | 40 |
Answer the following questions based on the above information:
A. If Sara used concentrated H2SO4 in place of dilute H2SO4, how many drops will be required for the change in colour to be observed?
(a) 40
(b) < 40
(c) >40
Justify your answer.
B. Sara measured 20 drops of dil. H2SO4 and found its volume to be 1 mL. If Sara observed a change in colour of NaOH solution by using 3 mL of H2SO4, how many mL of NaOH did she add to the test tube initially?
OR
Sara takes 10 drops of dilute H2SO4 in the test tube and adds two drops of phenolphthalein solution to it. Then she adds NaOH dropwise. Sara observes a change in colour after adding 20 drops of NaOH. What change in colour would she observe and why?
C. Write a balanced chemical equation for the reaction taking place in the above experiment. Which of the following is true and why? The reaction is a
(a) neutralisation and double displacement reaction
(b) neutralisation and precipitation reaction
(c) precipitation and double displacement reaction
(d) neutralisation, double displacement as well as precipitation reaction.
A. (b) < 40, because concentrated H2SO4 gives more H+ ions than dilute acid.
B. 3 mL of H2SO4 will be 60 drops, which will neutralise 6 mL of NaOH
| S. No. | Volume of dil NaOH taken (mL) | Drops of dil H2SO4 used |
| 1 | 2 | 20 (1 mL) |
| 2 | 3 | 30 (1.5 mL) |
| 3 | 4 | 40 (2 mL) |
| 4 | 6 | 3 mL = 60 drops |
OR
Colour will change from colourless to pink. Phenolphthalein in
colourless in acids and turns pink in basic solution.
C. 2NaOH + H2SO4 —> Na2SO4 + 2H2O
(a) neutralisation and double displacement reaction. Base NaOH is getting neutralised and forming salt + water. It is double displacement as Na+ ions are being replaced by H+ and OH by SO4 2- . It is not precipitation reaction because Na2SO4 is soluble in water.
29. Attempt either option A or B.
A. A hydrocarbon with the formula CxHy undergoes complete combustion as shown in the following equation: 2CxHy + 9O2 → 6CO2 + 6H2O.
(a) What are the values of ‘x’ and ‘y’?
(b) Give the chemical (IUPAC) name of the hydrocarbon.
(c) Draw its electron dot structure.
(d) Name the alcohol which on heating with conc. H2SO4 will produce the above hydrocarbon CxHy.
(e) Write a balanced chemical equation for the reaction of CxHy with hydrogen gas in presence of Nickel.
OR
B. The electronic structures of atoms P and Q are shown below
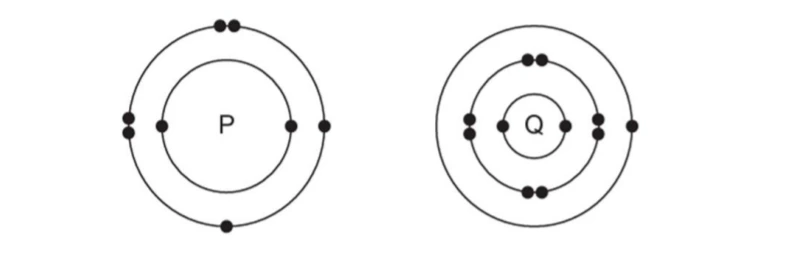
Based on the information given above, answer the following questions:
(a) If P and Q combine to form a compound, what type of bond is formed between them?
(b) Give the chemical formula of the compound formed.
(c) The compound so formed is dissolved in water. Is the resultant solution acidic or basic in nature? Justify your answer.
(d) Write the chemical equation for the reaction between ‘Q’ and ethanol.
(e) What will be the formula of the compound formed when ‘P’ undergoes bonding with carbon?
……………………………………………………..
For visually impaired students
A. A hydrocarbon with the formula CxHy undergoes complete combustion as shown in the following equation: 2CxHy + 9O2 → 6CO2 + 6H2O.
(a) What are the values of ‘x’ and ‘y’?
(b) Give the chemical (IUPAC) name of the hydrocarbon.
(c) Is CxHy a saturated or an unsaturated hydrocarbon?
(d) Name the alcohol which on heating with conc. H2SO4 will produce the above hydrocarbon CxHy.
(e) Write a balanced chemical equation for the reaction of CxHy with hydrogen gas in presence of Nickel.
OR
B. Oxygen can combine with both metals and non-metals. It combines with Calcium to form CaO and with carbon to form CO2.
(a) What type of bond is formed between carbon and oxygen?
(b) Identify the type of bond formed between Calcium and oxygen.
(c) Which of the above compounds will be a good conductor of electricity in molten state and why?
(d) Comment on the physical state (solid, liquid or gas) of CaO and CO2.
(e) What is the valency of carbon in CO2?
Student to attempt either option A or B.
A.
(a) x = 3, y = 6
(b) Propene
(c)
Student to attempt either option A or B.
A.
(a) x = 3, y = 6
(b) Propene
(c)
(d) Propanol
(e) C3H6 + H2 –Ni–> C3H8
CH2=CH-CH3 + H2 –Ni–> CH3-CH2-CH3
OR
B.
(a) Ionic bond
(b) Q2P
(c) Basic, metallic oxides are basic in nature.
(d) 2C2H5OH +2Q —-> 2C2H5OQ + H2
(e) CP2
For visually impaired students
A.
(a) x = 3, y = 6
(b) Propene
(c) Unsaturated hydrocarbon
(d) Propanol
(e) C3H6 + H2 –Ni–> C3H8
OR
B.
(a) Covalent bond
(b) Ionic bond
(c) CaO, due to presence of free ions in molten state.
(d) CaO is solid while CO2 is a gas.
(e) 4
Section C Physics
30. Arnav was making notes and he wrote down the following statements from his understanding of reflection from curved surfaces.
I. Concave mirrors can produce both real and virtual images depending on the position of the object.
II. Convex mirrors always produce real, inverted images regardless of the object’s position.
III. In both concave and convex mirrors, the image location can be determined using the mirror formula 1/f=1/v+1/u where f is the focal length, v is the image distance, and u is the object distance.Choose from the following the correct option that lists the correct statements about reflection from curved surfaces.
A. I and II
B. I, II and III
C. II and III
D. I and III
D. I and III
31. Choose the correct option from the below which explains the reason for us to perceive the day sky as blue.
A. As sunlight passes through the atmosphere, shorter wavelengths, such as blue are scattered more than other colors.
B. The sky appears blue because all colors are scattered equally, but blue light is stronger and more visible to the human eye.
C. The blue color of the sky is due to longer wavelengths like red and orange scattering more than shorter wavelengths, making blue stand out more.
D. The atmosphere contains blue-colored particles that give the sky its blue appearance.
A. As sunlight passes through the atmosphere, Rayleigh scattering causes
shorter wavelengths, such as blue and violet, to scatter more than other
colors, but our eyes are more sensitive to blue than violet.
32. Assertion (A): A point object is placed at a distance of 26 cm from a convex mirror of focal length 26 cm. The image will not form at infinity.
Reason (R): For above given system the equation 1/f=1/v+1/u gives v =∞
C. A is true but R is false
33.
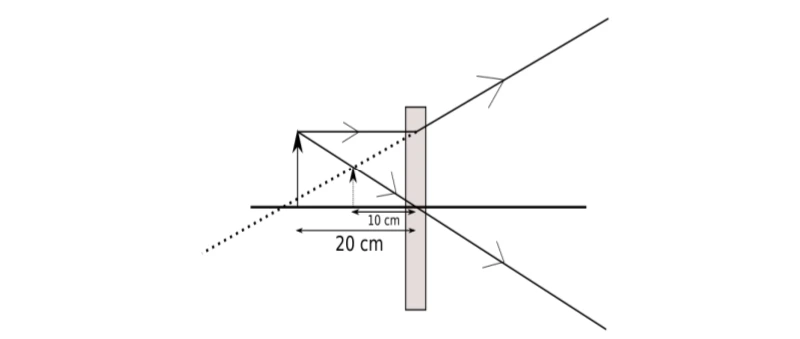
The above image shows the formation of an image with an optical instrument.
A. Identify the optical instrument (shown schematically as a rectangle) in the image.
B. What type of image is formed in this case?
C. Based on the measurements given in the image, calculate the focal length of the instrument.
……………………..
For visually impaired students
A. Under what conditions can a convex lens form a virtual image?
B. Why does a piece of paper catch fire if we allow sunlight to pass through a convex lens onto the paper?
A. The optical instrument shown in the figure is a concave lens.
B. The image formed is a virtual image.
C.
For visually impaired students
A. A convex lens can form a virtual image when the object is placed between the lens and its focal point.
B. A convex lens can focus parallel rays of sunlight to a single point, known as the focal point. Sunlight contains energy, and when this light is concentrated at a small point, the energy density increases significantly. This focused light energy raises the temperature at the focal point, which can become high enough to ignite a piece of paper placed at that point.
34. Attempt either option A or B.
Find out the following in the electric circuit given in the figure-
(i) Effective resistance of two 8 ohm resistors in the combination.
(ii) Current flowing through the 4-ohm resistor
OR
B.
Study the circuit and find out-
(i) Current in 12 ohm resistor
(ii) Difference in the readings of ammeter A1 and A2 if any
………………………….
For visually impaired students
A. You are given four resistors each having resistance of R ohm. Find the maximum and minimum resistance that can be made with these four resistors.
OR
B. A copper wire has a length L=2 m, a cross-sectional area A=0.5 mm2, and resistivity ρ=1.7×10−8 Ω-m.
Calculate the resistance of another wire made of the same material whose length is twice the length of the wire but has the same cross-sectional area.
35.
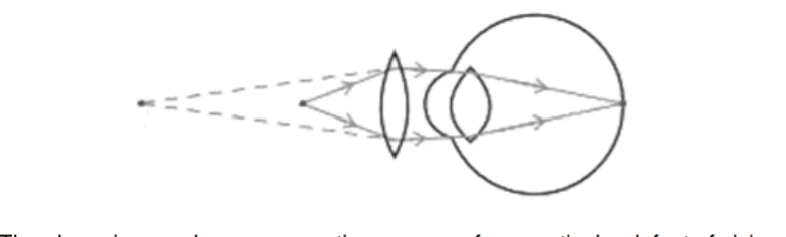
The above image shows a corrective measure for a particular defect of vision.
(i) Identify the defect of vision and state what kind of lens is used to correct this deficiency.
(ii) Draw and label a ray diagram that shows the defect of vision in the above case before correction.
For visually impaired students
(i) What is dispersion of light?
(ii) Explain the condition under which dispersion happens?
(iii) Give one reason that causes presbyopia
(i) Hypermetropia is the deficiency in vision and the lens is convex lens.
(ii)
For visually impaired students
(i) Dispersion of light is the phenomenon in which white light separates into its component colors (spectrum) when it passes through a medium, such as a prism. Different colours of light lend through different angles with respect to incident light, thus becoming district.
(ii) Dispersion occurs when light passes from one medium to another where the speed of light is different for each wavelength. For example, in a prism, each color of light has a different refractive index due to varying wavelengths, causing each color to bend at different angles as they exit the prism. Dispersion only happens if the medium has a variable refractive index across different wavelengths, like glass or water.
(iii) Presbyopia is caused by the gradual loss of flexibility in the lens of the eye, which occurs with aging. This reduced flexibility prevents the lens from changing shape effectively to focus on close objects, making it difficult to see them clearly.
36. A student needs to make a 0.12 Ω resistor. She has some copper wire of 0.80 mm diameter. Resistivity of copper is 1.8 × 10–8 Ωm
(i) Determine the cross-sectional area of the wire.
(ii) Calculate the length of wire required for the 0.12 Ω resistor.
37. Magnetic field lines are shown in the given diagram. A student makes a
statement that the magnetic field at X is stronger than at Y.
(i) Explain with reason if the student’s claim is correct.
(ii) Also redraw the diagram and mark the direction of magnetic field lines.
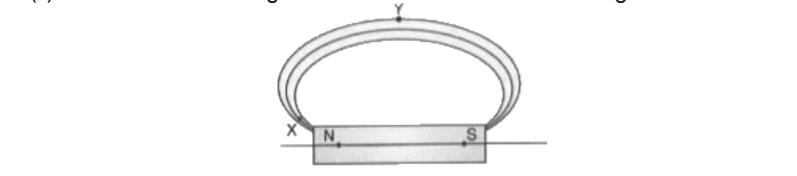
- Closeness of magnetic field lines is directly related to strength of magnetic field.
- Strength of magnetic field at point X (pole) is more than point Y.
- If the student redraws the diagram he/she should mark arrows correctly from North to South.
38.
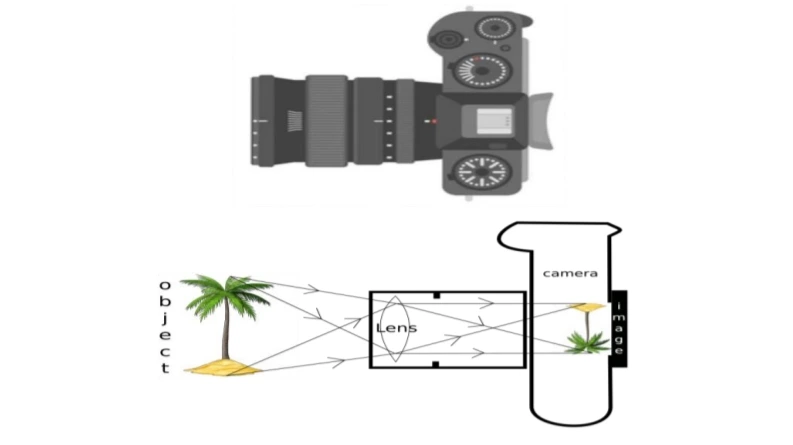
The above image is that of a Digital Single Lense Reflector (DSLR) Camera which are used to take high resolution photographs by professional photographers. The second image of the above two is a schematic diagram of how an image is formed on the sensor of the camera. Based on your understanding of the lenses, answer the following questions.
A. What type of lens is used in the DSLR camera shown in the image?
B. What type of image is formed on the sensor?
Attempt either subpart C or D.
C. A photographer is using a DSLR camera with a lens of focal length f=50 mm to take a close-up photograph of a small object. The lens projects an image onto the camera sensor that is located 60 mm behind the lens. Calculate the object distance (i.e., the distance between the object and the lens)
OR
D. A photographer is using a DSLR camera to take a picture of a flower. The flower is positioned 150 mm away from the camera lens. The actual height of the flower is 80 mm, and the image height formed on the camera’s sensor is measured to be 20 mm. Calculate the focal length of the camera lens.
For visually impaired students
Zarina worked as an apprentice in a factory where flashlights and solar cookers are made. She learnt to make the circuits, the design of the light-box and light concentrators of the solar cookers as well. She learnt the uses of lenses in making all those tools. Based on your understanding of lenses, answer the following questions.
A. What kind of lenses are used in the flashlight and light concentrator of the solar-cooker?
B. Give reasons for your choices in your answer for part A.
Attempt either subpart C or D.
C. An object is placed 40 cm away from a lens which is normally used in a solar-cooker. The image formed is twice the size of the object. Calculate the focal length of the lens.
OR
D. An object is placed 20 cm in front of a lens which is used in a flashlight, and the image is formed 10 cm away from the lens on the same side as the object. Calculate the focal length of the lens.
A. Convex Lens
B. Real and Inverted
Student to attempt either subpart C or D.
C. To find the object distance (u) for the lens, we can use the lens formula:
39. Attempt either option A or B.
A.
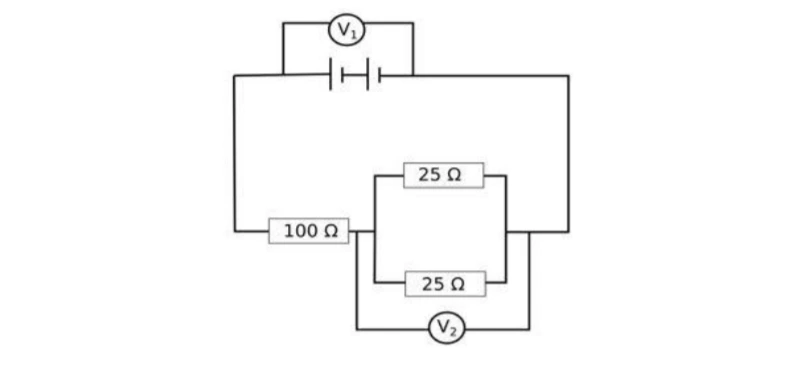
The arrangement of resistors shown in the above figure is connected to a battery.; The power dissipation in the 100 Ω resistor is 81 W. Calculate
(i) the current in the circuit
(ii) the reading in the voltmeter V2
(iii) the reading in the voltmeter V1
B.
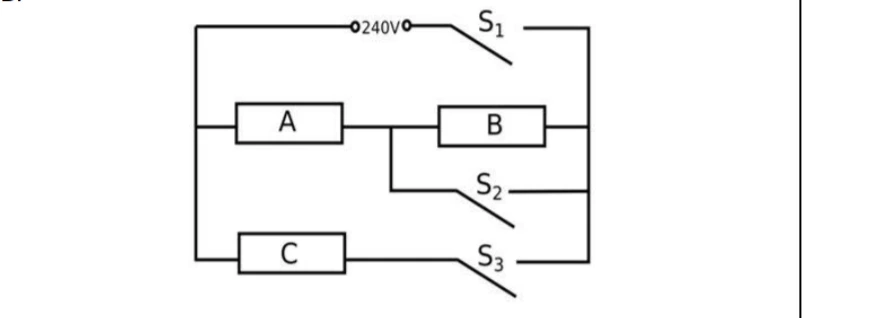
An electric heater consists of three similar heating elements A, B and C, connected as shown in the figure above. Each heating element is rated as 1.2 kW, 240 V and has constant resistance. S1, S2 and S3 are respective switches. The circuit is connected to a 240 V supply.
(i) Calculate the resistance of one heating element.
(ii) Calculate the current in each resistor when only S1 and S3 are closed. (iii) Calculate the power dissipated across A when S1, S2 and S3 are closed.
……………………………………….
For visually impaired students
A.
(i) Explain why in household circuits only the fuse is connected in series with all the rest of the appliances but all appliances are connected in parallel to each other.
(ii) In a household circuit, an electric heater of power 1500 Wand a fan of power 500 W are connected in parallel to a 220 V supply. A fuse rated for 10 A is connected to the circuit to protect it from excessive current.
(a) Calculate the total current drawn by the heater and the fan.
(b) Determine whether the 10 A fuse is appropriate for this circuit or if it will blow.
OR
B. Two resistors, R1=6 Ω and R2=12 Ω, are connected in parallel to a 24V battery. The circuit operates for 5 minutes.
(i) Calculate the total heat generated in both resistors.
(ii) If each resistor has a power rating of 100 W, determine whether it is safe to use these resistors in the circuit